Abstract
Background: Randomized control trials (RCT) have examined the safety and efficacy of direct oral anticoagulants (DOACs) compared to dalteparin in patients with cancer-associated thrombosis (CAT). Increased major bleeding (MB) was seen with edoxaban and increased clinically relevant non-major bleeding (CRNMB) was seen with rivaroxaban and there has been specific concern about increased risk of bleeding among patients with gastrointestinal malignancies (GIM). Results from RCTs comparing apixaban to dalteparin in CAT are pending.
Objective: Using the Mayo Clinic VTE registry, we examined the rates of MB, CRNMB, and overall bleeding (MB + CRNMB) in patients with GIM treated with anticoagulation for CAT.
Methods: Consecutive patients treated for CAT were enrolled into Mayo Clinic VTE Registry between March 1, 2013 and July 27, 2018 and were followed prospectively for safety and efficacy. Clinical, demographic and imaging data were collected from each participant at the time of study recruitment. Cancer type, stage, and treatment with chemotherapy were recorded. Major and CRNMB events were evaluated at 3-month intervals. Clinical status was assessed by written questionnaire in subjects unable to return at 3 months. Unreturned questionnaires were followed up with a scripted phone interview. The study was approved by Mayo Clinic Institutional Review Board.
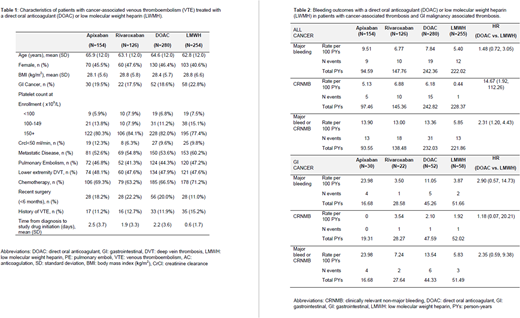
Results: In the 535 subjects in the registry with CAT, the mean age (64.6 vs. 62.8 years), mean BMI (28.4 vs. 28.8), and percent female (46.4% vs 40.6%) were numerically similar between DOAC and LMWH treatment groups (Table 1). Metastatic disease was present in 53.6% and 60.2% and chemotherapy was being administered in 66.5% and 71.2% of DOAC and LMWH treated patients, respectively. Thrombocytopenia (<100x109/L) at initiation of anticoagulation was present in 6.8% of DOAC treated and 7.5% of LMWH treated patients. Among all cancer patients, 110 (21%) patients had a GIM, 58 (53%) were treated with LWMH and 52 (47%) were treated with a DOAC (42% rivaroxaban or 58% apixaban). In all CAT, the rate of MB was similar in DOAC versus LMWH treated patients (7.8 vs. 5.4 per 100 person-years; HR 1.48, 95% CI 0.72-3.05; Table 2). The rate of CRNMB however, was significantly increased in the DOAC patients compared to LMWH treated patients (6.18 vs. 0.44 per 100 person-years; HR 14.7, 95% CI 1.92, 112.26). In the sub-group of patients with GIM, the rate of MB was non-significantly higher in the DOAC versus LMWH treated patients (11.1 vs. 3.9 per 100 person-years; HR 2.90, 95% CI 0.57, 14.73). In contrast to the overall CAT group, the rate of CRNMB was similar in the DOAC compared to LMWH treated patients (2.10 vs. 1.92 per 100 person-years; HR 1.18, 95% CI 0.07, 20.21). In GIM patients, apixaban compared to rivaroxaban had a numerically higher, but non-significant, rate of major bleeding (23.98 vs. 3.50 per 100 person-years; HR 4.0, 95% CI 0.54-29.87). In the overall group of CAT, the risk of major bleeding was similar between apixaban and rivaroxaban (9.51 vs. 6.77 per 100 person-years).
Conclusion: In our registry of subjects with CAT, we identified a significantly increased rate of CRNMB and overall bleeding, but not MB with the use of DOACs. In our data, GIM alone does not appear to account for the increased bleeding risk seen in CAT with DOACs, as we demonstrated no significant increase in major, CRNMB, or overall bleeding compared to LMWH. Direct comparison between apixaban and rivaroxaban was limited by sample size and number of events.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal